Protein Degradation
Building the Next Generation of Targeted Protein Degraders
Cullgen’s ubiquitin-mediated, small molecule-induced target elimination technology, uSMITE™, is used to develop targeted protein degraders for the treatment of perilous diseases, including cancer, inflammation, autoimmune diseases, and neurodegenerative diseases.
Traditionally, small molecule drugs are designed to modulate the functional sites of proteins and block their activity. Through the use of targeted protein degraders, we are able to expand the drug design paradigm beyond functional site inhibition – making it possible to eliminate previously “undruggable” enzymes and proteins.
Small molecule-mediated selective targeting of a protein represents an unprecedented opportunity in drug discovery and has several advantages over traditional drug discovery strategies, including:
High degree of target specificity that can be rapidly validated
Potential to reduce systemic drug exposure
Ability to counteract increased target protein expression induced by the inhibition of protein function
Small molecule-induced protein degradation is not limited (or even directed) to the functional sites of enzymes. Therefore, it can be applied to target proteins that are not currently therapeutically tractable, such as transcription factors, scaffold, and other non-enzyme regulatory proteins.
Degraders and the Ubiquitin-Proteasome System
-
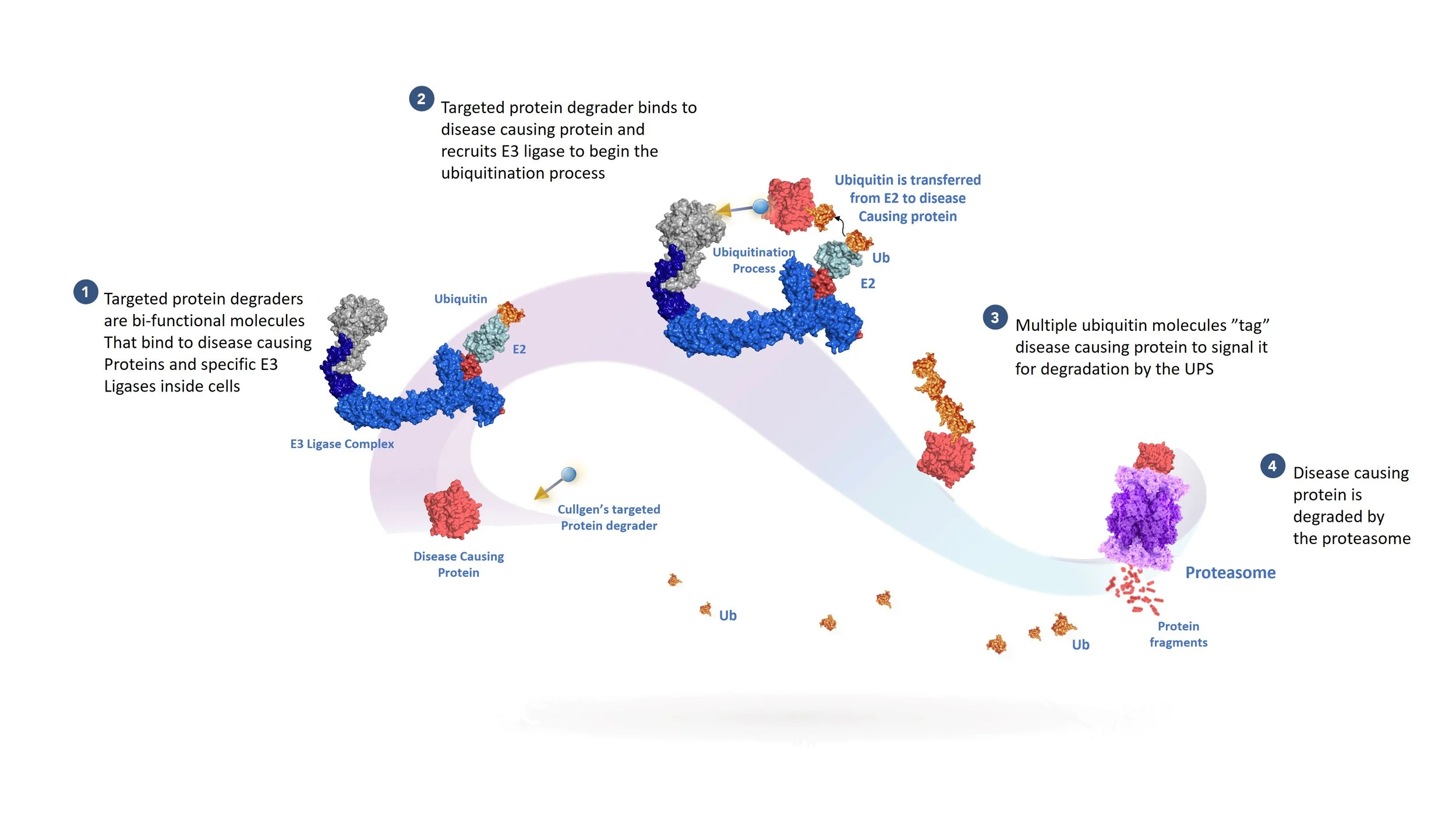
The Ubiquitin-proteasome system, or UPS for short, is the body’s natural way to remove unwanted or expired proteins. Protein degradation is initiated when an E3 Ubiquitin Ligase, together with E1 and E2 Ubiquitin ligases, marks the desired protein by adding a chain of small proteins called ubiquitins. The ubiquitin-tagged proteins are transported to the proteasome, degraded, and their building blocks recycled.
The UPS can also be used to remove disease-causing proteins by using targeted protein degraders, or degraders for short. Degraders can be used in multiple therapeutic areas, including cancer, inflammatory diseases, or neurodegenerative diseases. Degraders have two functional ends: one binds to the disease-causing protein, the other to an E3 ligase, bringing them together, starting the ubiquitin-tagging process and the degradation of the disease-causing protein by the proteasome. The degrader is released before degradation to target additional copies of the disease-causing protein in a catalytic fashion.
Learn more at Cullgen.com
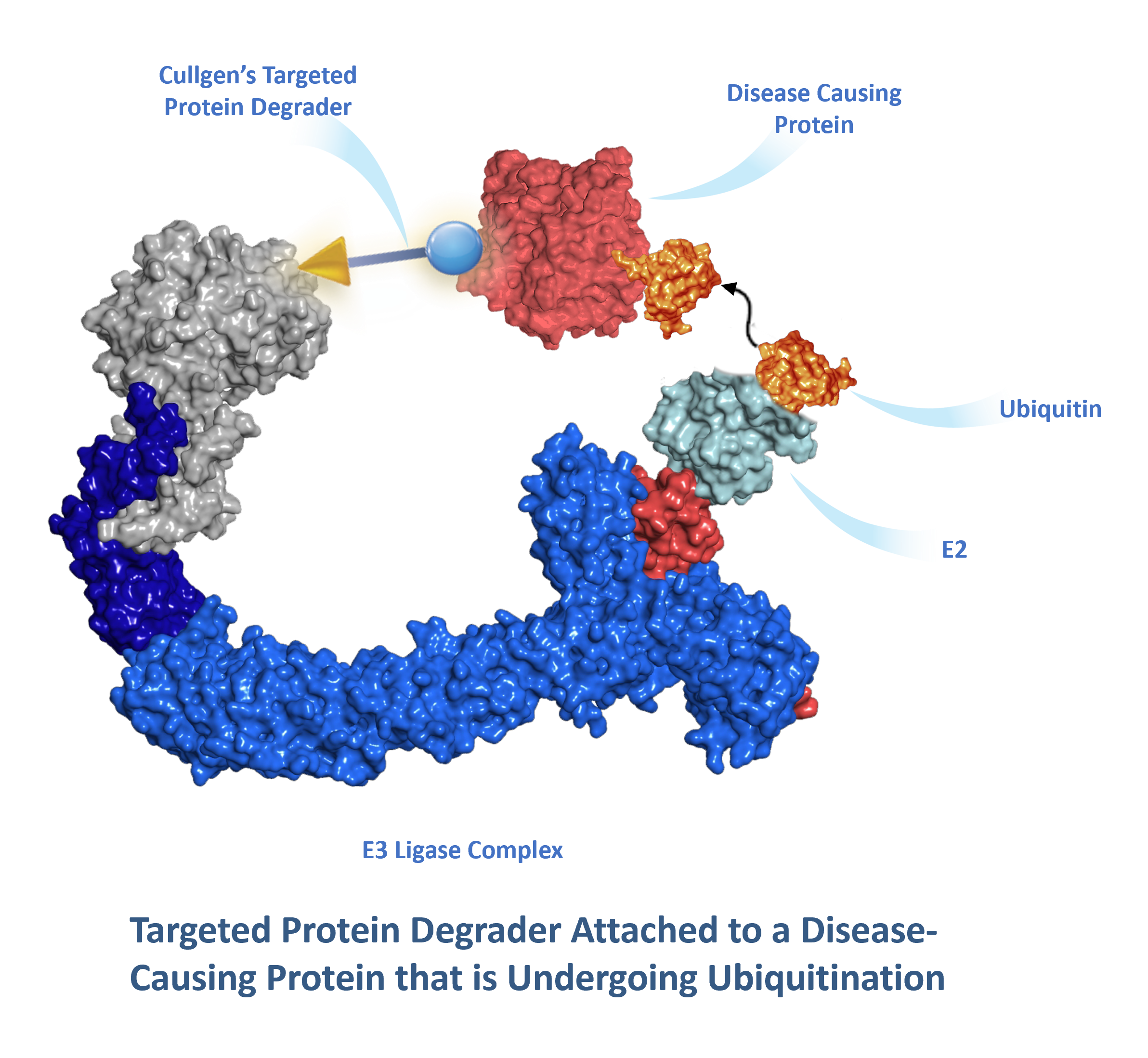
How A Targeted Protein Degrader Works
Scientists at Cullgen have developed small molecules, known as targeted protein degraders, that have two functional ends connected to one another by a linker. One end of the degrader contains a ligand (the “warhead’) that binds to a specific disease-causing protein that will be targeted for destruction and the other end, the E3 ligand, recruits and binds to an E3 ligase complex.
The degrader molecule is typically adminstered to a patient orally and is distributed to cells containing the disease-causing protein. After the warhead portion of the degrader binds to the disease-causing protein, the E3 ligand portion of the degrader binds to a specific E3 ligase that utilizes the ubiquitin-proteasome system (UPS) to initiate the process of poly-ubiquitination of the disease-causing protein. You can learn more about the UPS and different E3 ligases in detail here.
Once the disease-causing protein has been sufficiently ubiquitinated, it is recognized by the proteasome for destruction.